By Paula Hsiao
The Industrial Revolution has brought on more than just rising global temperatures, melting ice caps, and the normalization of environmental degradation. A less commonly discussed effect of the increased amounts of carbon dioxide pollution was ocean acidification. It’s often overlooked or ignored because humans are a lot like ostriches. If we can’t see something, we assume it can’t see us. In this case, since we can’t see the immediate effects because the changing ocean chemistry is happening under, we are oblivious to the drastic repercussions it will lead to. A quarter of the carbon dioxide that’s released in the air does not actually stay in the air, but instead, dissolves into the ocean. This means that since the Industrial Revolution, the ocean has absorbed about 525 billion tons of carbon dioxide. Although this might mean there’s less carbon dioxide in the air, thus preventing the gradual warming of our planet, it has begun to change the ecosystems and biodiversity of the oceans through changes in ocean chemistry. As more carbon dioxide is dissolved into the sea, it causes the ocean to become acidic because there is more hydrogen ions released into the ocean (the concentration of hydrogen determines acidity). This is why the ocean has become more acidic after the Industrial Revolution since the new factories, cars, and coal plants all started polluting more carbon dioxide into the air, and therefore, the oceans. The acidity of oceans has already increased by 30% in the past two centuries.
How does ocean acidification occur?
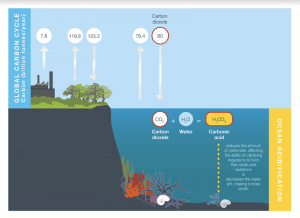
When carbon dioxide enters the oceans, it combines with H2O to make carbonic acid. Seeing as the result is an acid, it will fall apart like all acids do, causing it to lose hydrogens. The carbonic acid breaks down into bicarbonate after losing a positive hydrogen ion and then carbonate after losing another positive hydrogen ion. The carbonate usually combines with the calcium in the seawater, which neutralizes the carbonate and forms calcium carbonate (limestone), useful for the skeletons of marine animals like coral. All these chemical reactions are reversible, however, during Pre-Industrial Revolution times, there was a net positive in the amount of calcium carbonate being produced compared to that of being separated back into carbonate and calcium. After the Revolution, there was now a net positive of calcium carbonate being produced, which negatively affected marine ecosystems.
Effects on Calcifiers
Organisms that have shells or skeletons made of calcium carbonate are called Calcifiers, and are one of the most abundant forms of life in the oceans. Calcifiers range from plankton that form the basis of the food chain to the coral reefs expanses that are the homes to many marine organisms. Since ocean acidification will reduce the amount of calcium carbonate available to the organisms, they spend more energy producing shells, leaving them too tired to find food, reproduce, or to defend against predators. This means that populations of species could go extinct or at least heavily decline as ocean acidification persists.
Plankton species like pteropods are food for many marine organisms like fish, whales, and seals. However, it isn’t just about the ability to make shells. The calcium carbonate shells that calcifiers already have can be very sensitive to increased ocean acidification (lower pH levels). In an accidental experiment by Victoria Fabry, she found that within 48 hours of placing pteropods in seawater with the pH level of the Southern Ocean by 2100, the shells had begun to dissolve.
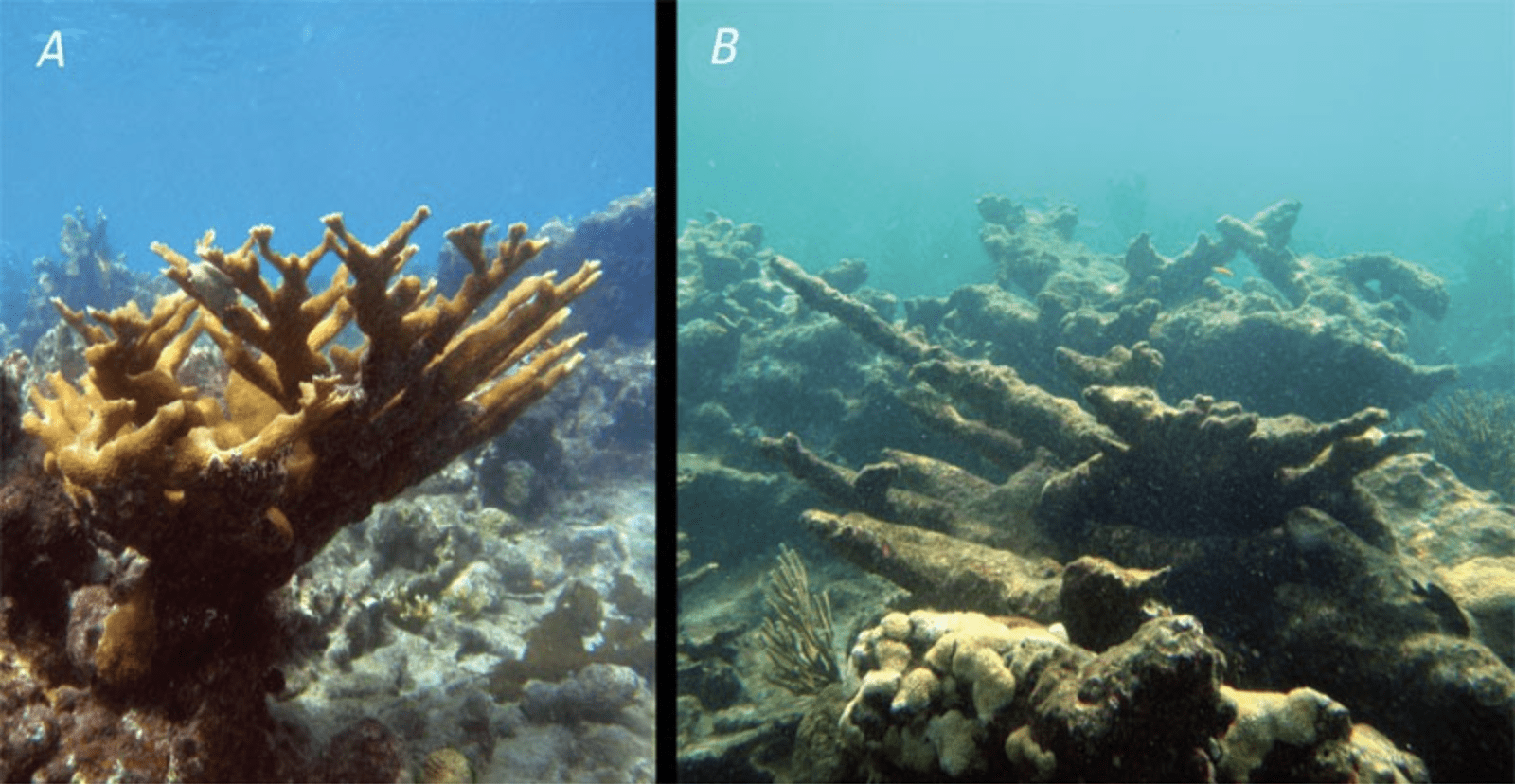
Coral Reefs
The Great Barrier Reef of Australia has seen a growth rate decrease of 14% since 1990. This is a rapid decline of an ecosystem that is the largest biological structure in the world and home to more than 1,500 marine organisms. As the growth rates continue to plummet, the reef itself will start to shrink. Ocean acidification is perhaps to blame for this decline as reefs rely on carbonate ions to form shells made of calcium carbonate. However, since ocean acidification takes away the availability of carbonate, it hinders the ability of coral to repair or grow their shells. Other contributing factors in coral reef decline include rising ocean temperatures and pollution, but the combination of all these will seriously endanger the health of the coral reefs and the marine ecosystem as a result.
Our “Keeling” Environment
Charles Keeling was best known for his chart, the Keeling Curve. It maps precise measurements of atmospheric carbon dioxide throughout the years. The measurements depict the steady rise of carbon dioxide levels through a 47 year period of time. Sometimes, there are fluctuations caused by plant growth absorbing carbon in the summer/spring, and releasing carbon in fall/winter. This is the world’s longest continuous record of atmospheric carbon dioxide and was the first to confirm that atmospheric carbon dioxide was caused by fossil fuels burning. The graph has shown an overall upwards linear trend of carbon dioxide use. If carbon dioxide emissions continue to rise at these rates, it will not only greatly affect the lives of the marine life who are subjected to ocean acidification, but also has repercussions on coastal communities that depend on fishing or tourism. The only real way to mitigate ocean acidification is to reduce carbon emissions and our use of fossil fuels.